FLD-103: A Non-Surgical Treatment for Basal Cell Carcinoma with the Potential to Change Lives
Basal cell carcinoma (BCC) is the most common type of skin cancer and the most frequent form of all cancers. In North America, 3.6 million cases are diagnosed each year. BCC results from the uncontrolled growth of skin cells, primarily in areas of the body that have been exposed to the sun for extended periods. Patients affected by BCC commonly develop tumors on the face, neck, scalp and arms.
The standard treatment for BCC is surgical removal or excision. While non-surgical treatments exist, they are not indicated for all types of BCC and often show limited efficacy. As a result, many patients must undergo surgery, facing significant preoperative anxiety, psychological distress, and lengthy recovery periods. Mohs surgery is a commonly used technique, that involves cutting away layers of skin until there is no evidence of cancer. This procedure results in wounds that can frequently take months or even years to heal and can leave permanent scars on visible areas of the body. Additionally, patients with BCC are at a higher risk of developing new or multiple lesions, requiring repeated surgeries.
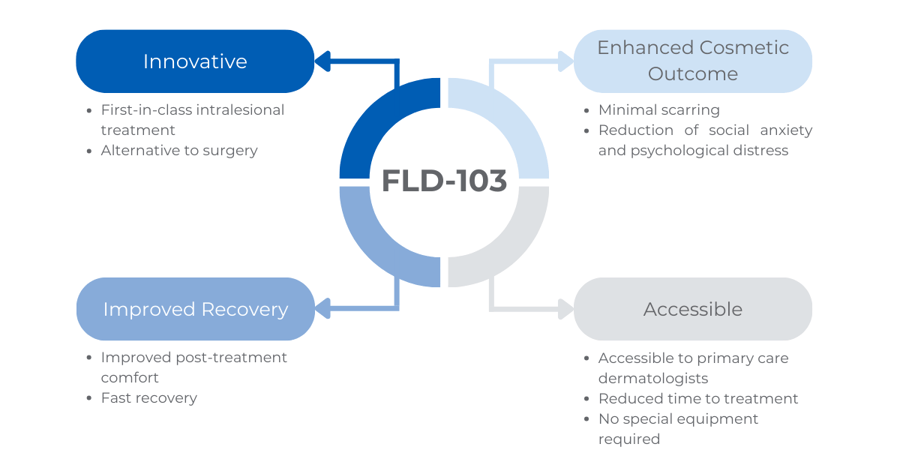
Feldan Therapeutics is developing FLD-103, an efficient, non-surgical approach to treat BCC. This first-in-class intralesional treatment selectively targets BCC cells by disrupting an intracellular component involved in the pathophysiology of the disease. By minimizing side effects, avoiding scarring and the need for postoperative recovery, FLD-103 has the potential to become a non-surgical first-line option and life-changing treatment for patients suffering from BCCs.