The Feldan Shuttle:
Breaking the Endosomal Barrier to Reach Untapped Therapeutic Targets
The Feldan Shuttle: Optimized for Functional, Safe, and Broad Delivery
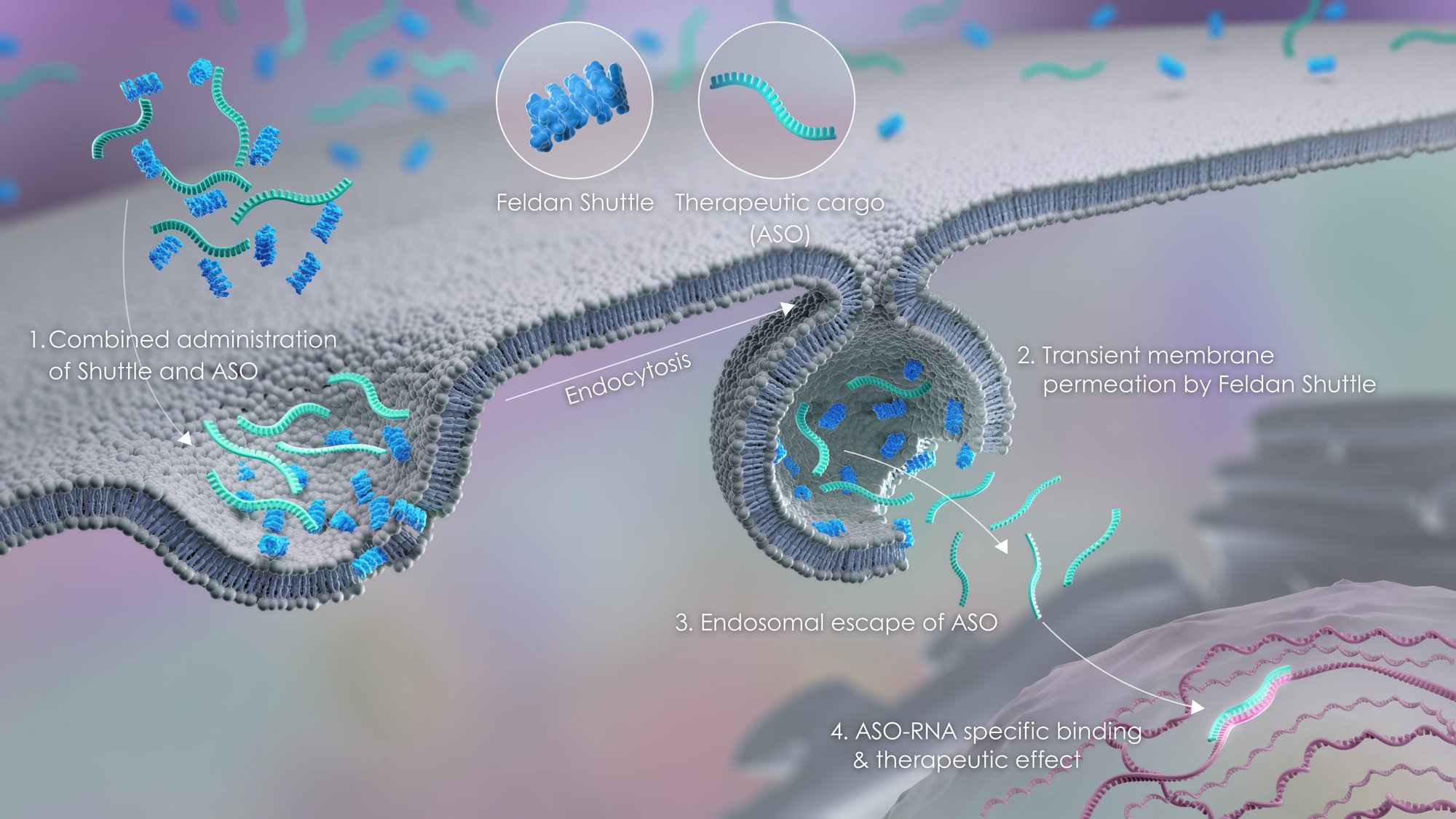
Overcomes endosomal entrapment — a major obstacle to effective intracellular drug delivery
Enables local delivery of functional antisense oligonucleotides (ASOs) and other therapeutic cargos
Achieves rapid delivery into a wide range of cells and tissues, including the skin and lungs — two organs known for their challenging natural barriers that limit traditional delivery methods

With a robust safety profile demonstrated in preclinical studies, the Feldan Shuttle led to the advancement of our lead candidate, FLD-103, into clinical trials

Unlocking the Full Potential of Antisense Oligonucleotides with the Feldan Shuttle
The Power of Local Administration
Our pipeline focuses on therapies administered directly at the disease site which offer key advantages to improve therapeutic outcomes:
Increases
Treatment Efficacy
Limits
Systemic Exposure
Improves
Safety Profile
Manufacturing Insight
Both the Feldan Shuttle peptide and the ASO are chemically synthesized through an established and readily scalable manufacturing process.
Publications
The Feldan Shuttle has been the subject of publications in renowned journals, demonstrating its ability to efficiently deliver therapeutic cargos into cells, where they can engage their intracellular targets. This peer-reviewed work — some developed in collaboration with academic partners — reflects our scientific engagement and our platform’s broad potential.



